PreSlice: Depth Guide for Skin Biopsies
- Tanishk Sinha
- Ellie Zhang
- Alp Demirtas
- Katie Liang
- Samhita Vasu
- Iris Kwon
- Ria Thakur
- Christian Guaraca
- Michelle Zwernemann (Faculty mentor)
- Dr. Elise Ng (Clinical mentor)
- Dr. Kristin Bibee (Committee member)
- Dr. Jaryslow Jedrych (Committee member)
- Alissa Murphy (Committee member)
- Matthew Shaeffer (Committee member)
- Leanne Pichay (Teaching assistant)
Abstract:
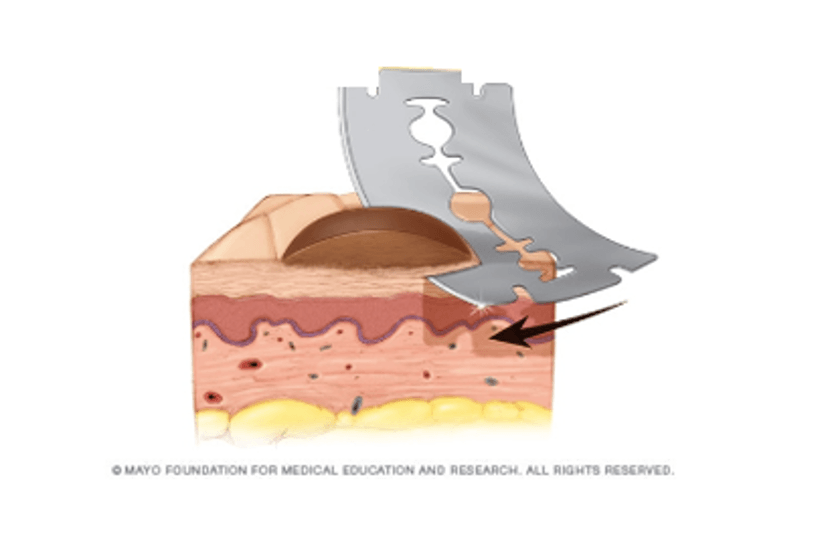
Melanoma is the deadliest form of skin cancer. Though highly treatable in its earlier stages, survival rates drop significantly once metastasis has occurred. Treatment is guided by staging, obtained primarily by the depth the melanoma invades into the skin. Accurate melanoma staging relies on a skin biopsy specimen with the entire depth of the lesion. With over 79,000 melanoma diagnoses per year in the US, the ability to obtain specimens that provide this prognostic information is critical. Shave biopsies, the most common biopsy method, utilize a simple razor blade to shave off lesions, providing a quick and affordable method for extraction. However, because biopsy blades contain no mechanism to control the skin depth extracted, up to 43% of biopsies result in transection and nearly 13% of melanoma cases are incorrectly staged. A method for extracting skin biopsies at precise depths would drastically reduce the transection rate and subsequently increase the accuracy of patient melanoma staging.
To address this need, the PreSlice device has been designed with a preset depth guide that standardizes the extraction depth of skin biopsies. PreSlice is designed to optimize shave biopsy depth precision between 1-4 mm, the average depth of melanoma lesions; to prevent inaccuracy in depth extraction over .25 mm to mitigate incorrect staging; and to maintain the affordability and convenience of traditional shave biopsies. Prototypes were tested via mock skin biopsies on grapefruit albedos, a standard clinical practice, and validation studies were performed with the assistance of clinicians from the Johns Hopkins Hospital. Initial results confirm the utility of a proof-of-concept PreSlice device for biopsy precision and clinical workflow integration.