PneuTech: Increasing the Safety of Percutaneous Lung Biopsies
- Katia Kovrizhkin
- Sean Darcy
- Ashley Tsang
- Jacob Desman
- Deborah Weidman
- Tatiana Pereira
- Gohta Aihara
- Robert Liddell, PhD
- Shababa Matin
- Harjit Singh, MD
- Rich Middlestadt
- Supriya Munshaw, PhD
- Bibhav Poudel
- Kimberley Studeman, MD
- Ian McLane
- Sarah Lee
Abstract:
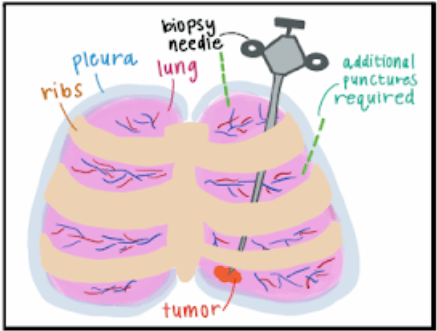
A percutaneous core needle lung biopsy is a minimally invasive procedure by which a sample of abnormal lung tissue is obtained through a needle inserted directly into the targeted site of the lung using imaging guidance.The most common complication during this procedure is pneumothorax, a collapsed lung, which occurs in 1 in 5 patients. With over 100,000 procedures performed in the United States alone, pneumothorax contributes to over $120 million in annual hospital costs. Thus, there is a critical need to improve the safety of percutaneous lung biopsies. The current standard of care utilizes a straight needle guided by computed tomography (CT), making it a challenge to direct the needle to the target site while navigating around critical structures, such as major blood vessels, airways, and ribs (Figure 2). Therefore, additional pleural punctures within a single biopsy or a repeat biopsy may be necessary to obtain an adequate sample, increasing the likelihood of pneumothorax. Our solution addresses these issues via a novel needle system equipped with the ability to access any target site in any location while minimizing the number of pleural punctures. By enabling safer high-yield lung biopsy procedures, our device has the potential to revolutionize the lung biopsy space.