BME Associate Professor Warren Grayson is shaping a revolutionary solution that will change the lives of millions who have had their faces disfigured by a genetic disorder, ravaged by cancer, or shattered in a car accident.
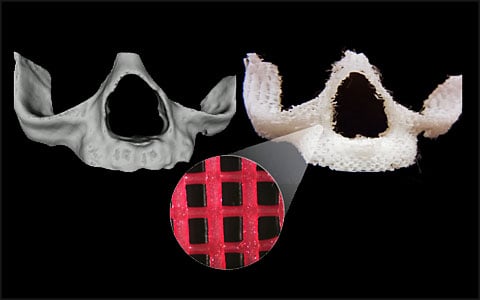
Grayson’s approach to craniofacial bone reconstructive surgery combines 3-D printing, cell signaling techniques, and a patient’s own stem cells to engineer a living, anatomically precise facial bone.
Today, surgeons doing reconstructive facial surgery rely on the materials at hand. As an example, to build the jaw, they may harvest bones from elsewhere in a patient’s body, often a fibula — the thinner of two bones in the lower leg. They break the fibula into four pieces, chisel them to fit, and rearrange them to rebuild the jaw.
For Grayson, the best solution to rebuilding faces is to stimulate the body’s own regenerative abilities to “grow” new anatomically shaped bone from engineered bio friendly materials. Patients would come to Johns Hopkins for facial reconstructive surgery with their custom bone, or scaffold, ready for implant. The implant would then gradually dissolve as living tissue forms.
This interdisciplinary approach would be implemented by Johns Hopkins surgeons and engineers. The vision for the new approach would entail having:
- Surgeons scan a patient’s face using CT and transmit the data to tissue engineers.
- Tissue engineers would use a 3-D printer to produce a porous, biodegradable bone “scaffold” made from ground-up bone and embedded tiny beads designed to release precise levels of oxygen that direct stem cell growth.
- Within 12 to 24 hours, the custom bone scaffold would arrive at the operating room in a sterile package.
- Surgeons would coat the scaffold with stem cells extracted from the patient’s fatty tissues and implant the tissue-engineered bone into the patient.
- The regeneration process begins, and in time the implanted scaffold would dissolve and be completely replaced with living regenerated bone and tissue.
This improved facial reconstruction methodology will literally transform the lives of many, however, challenges remain.
Persuading stem cells to make the kind of tissue required in just the right shape and at just the right time is tricky. In deciding what kind of tissue to become, stem cells respond to subtle environmental and chemical signals, such as oxygen and calcium levels. Higher oxygen encourages bone growth, while lower oxygen can trigger the development of blood vessels. Both are needed to build a new bone.
There are also regulatory hurdles. So far, the U.S. Food and Drug Administration has not approved any stem cell-based products for use, other than blood-forming stem cells harvested from the umbilical cord.
Grayson is finding support and funding for his investigative studies. Last year he was selected to receive a Johns Hopkins Catalyst Award to help faculty kick start their research initiatives and creative endeavors. He also was awarded a Maryland Stem Cell Research Grant.
To provide the infrastructure for this approach, Grayson is in discussions with colleagues about creating an institute for craniofacial reconstruction, which would include a 3-D printing facility and other labs for preparing facial bone implants for surgery.
As Grayson tries to orchestrate the science and funding for his vision, he says he tries not to lose sight of the ultimate goal. Every day, he says, he and his research team “never forget why we do the research.”