The Yin and Yang of calmodulin revealed by a biological signal generator
Probing biological networks with custom-shaped signaling inputs has long been a coveted dream of engineering-minded biologists, keenly aware of the power of analyzing man-made circuits with an electrical signal generator. Researchers in the Calcium Signals Laboratory at Johns Hopkins University have devised such a generator for the delivery of calmodulin, the most widespread Ca2+ sensing molecule throughout the biological kingdom. When this calmodulin device was applied to ion channels, molecules controlling vital bioelectricity in the heart and brain, a surprising realm of calmodulin activity was revealed.
These advances are described in the featured article in the 23 October 2014 issue of the journal Cell. The research paper “Apocalmodulin itself promotes ion channel opening and Ca2+ regulation,” was written by lead author Paul Adams, along with co-authors Manu Ben-Johny, Ivy Dick, synthetic biologist Takanari Inoue, and Hopkins Biomedical Engineering Professor David T. Yue.
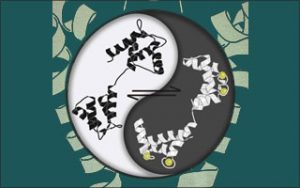
In particular, the ebb and flow of free ionized Ca2+ within cells serves as a language of life — coordinating a multitude of critical processes within cells. On this stage, the Ca2+-free form of calmodulin (shown on the left “Yin” side) has often been viewed as dormant, simply awaiting an upsurge of Ca2+ions to transform it into an action-packed, Ca2+-bound form of calmodulin (shown on right “Yang” side). For example, this Ca2+-bound calmodulin has been known to throttle the opening of these ion channels (condition of weakness, or Yin) to maintain good Ca2+and electrical rhythms under normal conditions, or stave off bad rhythms in disease.
By applying the calmodulin generating device to ion channels, the Hopkins team revealed that Ca2+-free calmodulin is in no way dormant, but instead markedly boosts their opening (condition of strength, or Yang). In fact, the conversion of calmodulin to its Ca2+-bound form simply relieves this initial enhancement in opening. Thus, it is the dynamic interplay of Ca2+-free calmodulin (over white Yang domain) and Ca2+-bound calmodulin (dark Yin domain) that controls the opening of ion channels, depicted in the background.
The implications are enormous. This Yin-and-Yang dialectic between the two forms of calmodulin opens exciting new strategies for treating diseases relating to maladaptive bioelectrical and Ca2+ signaling, such as cardiac arrhythmias, epilepsy, and Parkinson’s. This dual-faced mechanism will likely be relevant to many other molecules besides ion channels. With biological signal generators in hand, more discoveries loom on the horizon.
