“Despite its importance, metastasis is not well understood, and the drugs still don’t work well enough,” Ewald says.
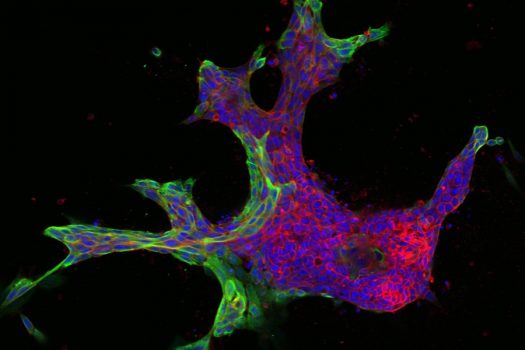
The research relies on live tissue from volunteer breast cancer patients at The Johns Hopkins Hospital, which Ewald’s lab uses to generate its 3-D organoids. Each sample can yield thousands of organoids derived from different parts of the tumor, reflecting a complex spectrum of mutations, gene expression, and invasive behavior.
From there, Bader’s computer models classify the organoids numerically, matching the varying shapes with their molecular drivers. He starts by ordering the organoids from the simplest to the most complex shapes—beachball-like versus starfish, for example—to rank them from least to most invasive.
Overall, the goal is to zero in on mutations and gene expression patterns that allow breast cancer to spread to distant organs. Once these key molecules are identified, the researchers say, better drugs can be developed to disrupt metastasis.
“We’re trying to discover new molecular targets that are important for cancer at different stages of metastasis—for invasion, dissemination, and regrowth,” Bader says.
On the clinical end, three Johns Hopkins Medicine faculty members—David Euhus, Ashley Cimino-Mathews, and Edward Gabrielson—help patients donate their tumor samples as part of the surgical process.
The research builds on Ewald’s 15 years working with organoids, “trying to make metastasis something we can understand in the lab,” he says. For Bader, a theoretical and computational scientist with expertise in genomics, the collaboration has marked his first venture into cancer research.
The two first joined forces in 2012 to identify molecular drivers of cell escape, then continued to study how groups of cancer cells spread through the body. Their early collaborations won grants from the American Cancer Society and the Jane Koskinas Ted Giovanis Foundation to develop the cancer organoids and the mathematical approaches respectively, paving the way for the recent National Cancer Institute grant.
“We’re continuing to work toward a better understanding of metastasis,” Ewald says. “Once these big questions are unlocked, we’ll have a much better shot at improving patients’ outcomes with breast cancer.”
-Katie Pearce
This article first appeared on the Hub.