New immune model sheds light on implant rejection
Implantable medical devices–think artificial joints, cochlear implants, and insulin pumps–make some of our most challenging health issues more manageable. Even so, human bodies frequently reject implanted biomaterials, and currently, there are few ways to predict or prevent it from happening.
A team of researchers led by Joshua Doloff, an assistant professor of biomedical engineering, has discovered an advanced “humanized” mouse model to better predict how the human immune system will react to medical implants. Their results appear in Science Advances.
“By directly studying how human immune cells interact with implants in a mouse, we can unlock a much deeper level of mechanistic understanding of implant rejection responses,” said Doloff. “It’s our hope long-term that this newly identified humanized mouse model variant will be useful for expanding preclinical screening results so that implants have a higher rate of translational success into patients.”
In addition to Doloff, biomedical engineering graduate students Victor M. Quiroz and Amanda Rakoski also contributed to the study.
Humanized mice—mice that are treated with radiation to remove their intrinsic mouse immune system and then injected with human cells that give rise to human-like immune systems—are used to study many medical conditions and gain insights that would otherwise require an invasive procedure in a human subject.
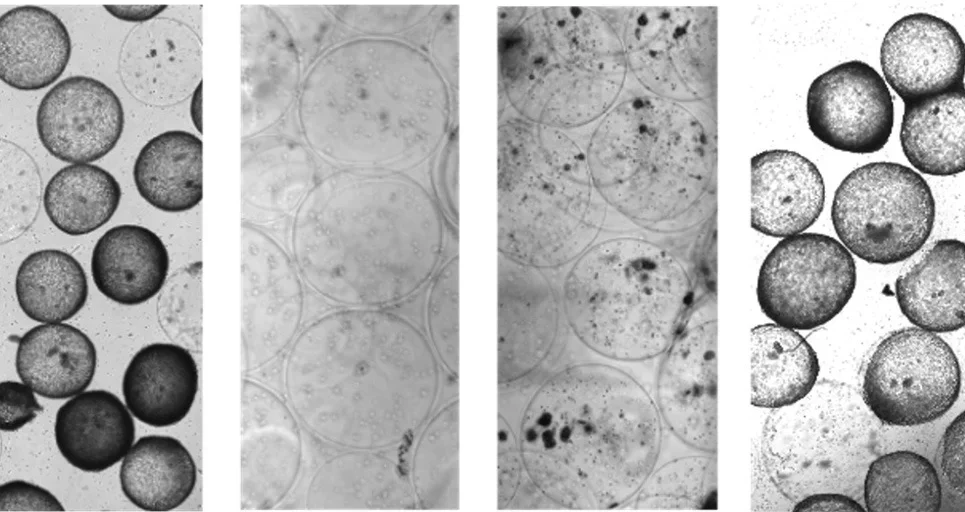
In the new study, Doloff’s team reports the first humanized mouse model variant capable of investigating why scar tissue forms around an implanted device, a process known as fibrosis or foreign body response. In some cases, fibrosis can cause pain to the patient and even damage to an implant, making it ineffective.
The team faced a significant hurdle as previous studies had demonstrated the failure of several versions of humanized mouse models to develop biomaterial-induced fibrosis.
“The human immune system comprises two distinct ‘arms’ that work together to defend the body against disease and infection: the innate arm and the adaptive arm,” Doloff said. “When we evaluated some of these previous humanized models, we noticed that while the mice had developed appropriate levels of adaptive immune (T & B) cells, they were missing the entire innate arm of their immune system. This presented us with a challenge because the innate arm contains the cells that are necessary for fibrosis to occur, namely ‘macrophages.’”
To overcome this, Doloff’s team genetically modified the mice to express three additional human cytokines: signaling molecules that play a crucial role in stimulating an innate immune response. Next, they inserted different material implants, either hydrogel or plastic polymers like silicone, into the mice.
It worked. Using this new mouse model and humanized variant, the researchers could see the early development and progression of fibrosis, including which human immune cell types were driving the condition and how they were localizing around the implant. According to Doloff, the model seems particularly valuable because it provides further evidence that macrophages are in fact essential to fibrosis: when those cells are missing, scar tissue does not form around implanted devices.
The team also observed what they deem “crosstalk” between human macrophages and mouse fibroblasts, the cells that make scar tissue.
“Although macrophages and fibroblasts are known to be key players in fibrosis, our model allows the opportunity to reveal more about how human immune cells interact with both the implant as well as downstream players in regulating this process,’ said Doloff.
The team believes the model will open new avenues of study; for example, future work may build on these results to understand how specific biomaterials and implant sites affect fibrosis.
By uncovering the cellular dynamics at play in a mouse model, the researchers hope to translate these findings to human applications, such as generating predictive, perhaps even personalized, tools for understanding and preventing fibrosis and implant rejection.
“In the future, it may be possible to predict the host response to an implanted biomaterial and even intercept implant rejection at early stages of its development in patients,” said Doloff.
