Low-cost tissue-freezing device could expand access to lifesaving breast cancer treatments
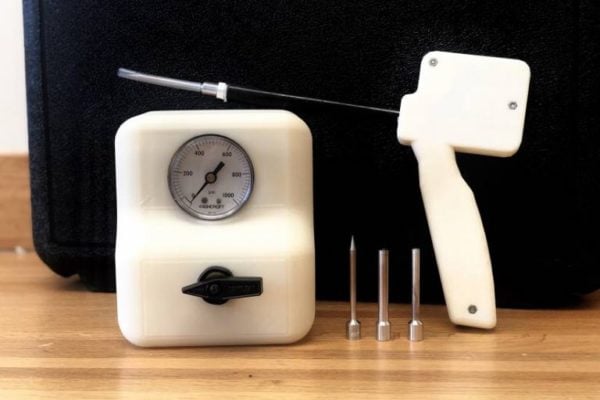
A reusable breast cancer treatment device created by a group of students at Johns Hopkins University offers a low-cost alternative for women in low-income and low-resource countries.
The tissue-freezing probe uses cryoablation, a method that kills cancerous tissue by exposing it to extremely cold temperatures, and employs carbon dioxide, a widely available and affordable alternative to argon, the current industry standard.
A study detailing the tool’s success in animal studies was published this month in PLOS One.
“Innovation in cancer care doesn’t always mean you have to create an entirely new treatment,” says Bailey Surtees, a recent Johns Hopkins University biomedical engineering graduate and the study’s first author. “Sometimes it means radically innovating on proven therapies such that they’re redesigned to be accessible to the majority of the world’s population.”
While the survival rate for women with breast cancer in the United States is greater than 90%, it is the largest cause of cancer-related mortality for women across the globe and disproportionately affects women in lower-income countries, where treatment options are scarce. Survival rates for women with breast cancer in Saudi Arabia, Uganda, and The Gambia are just 64%, 46%, and 12%, respectively.
“Instead of saying ‘she has breast cancer,’ the locals we met while conducting focus groups for our research said ‘she has death,’ because breast cancer is often considered an automatic death sentence in these communities,” says Surtees.
In lower-income countries, the main barriers to treating breast cancer are inadequate treatment options. Surgery, chemotherapy, and radiation are often impractical or too expensive, and women in remote areas have long travel times to regional hospitals. Even if a woman is able to travel to a hospital for treatment, she may not be seen, and recovery times will keep her out of work for an additional few weeks.
Cryoablation is an optimal treatment option in these countries because it eliminates the need for a sterile operating room and anesthesia, thus making it possible for local clinics to perform the procedure. It’s also minimally invasive, thereby reducing complications such as pain, bleeding, and extended recovery time.
Current cryoablation technologies, however, are expensive, with a single treatment costing more than $10,000. The devices rely on argon gas, which typically isn’t available in lower-income countries, to form the tissue-killing ice crystals.
With these barriers in mind, the student-led research team, named Kubanda—which means “cold” in Zulu—wanted to create a tissue-freezing tool that uses carbon dioxide, which is already widely available in most rural areas thanks to the popularity of carbonated drinks.
The research team tested its tool in three experiments to ensure it could remain cold enough in conditions similar to the human breast and successfully kill tumor tissue.
In the first experiment, the team used the tool on jars of ultrasound gel, which thermodynamically mimics human breast tissue, to determine whether it could successfully reach standard freezing temperatures to kill tissue and form consistent ice balls. In all trials, the device formed large enough ice balls and reached temperatures below 40 degrees below zero Celsius, which meets standard freezing temperatures for tissue death for similar devices in the United States.
For the second experiment, the team treated rats with mammary tumors. Afterwards, team members looked at the tissue under a microscope and confirmed that the tool successfully killed 85% or more tissue for all tumors. In a third animal experiment, the device was shown to be capable of staying cold enough during the entire experiment to kill the target tissue.
“When we started the project, experts in the area told us it was impossible to ablate meaningful tissue volumes with carbon dioxide,” says Nicholas Durr, an assistant professor in the Department of Biomedical Engineering at Johns Hopkins and the study’s senior author. “This mindset may have come from both the momentum of the field and also from not thinking about the importance of driving down the cost of this treatment.”
While the results are promising, the device still requires additional testing before it’s ready for commercial use. The research team’s next steps include ensuring the device can consistently kill cancer tissue under the same heat conditions as human breast tissue.
In the near future, the team hopes to continue testing its device for human use and expand its use to pets.
The device has been in development by students and researchers at Johns Hopkins since 2015. It debuted in 2016 at Design Day, an annual showcase of projects designed and constructed by undergraduate and graduate students in the Department of Biomedical Engineering.
“This project is a remarkable example of success from the Biomedical Engineering Design Program,” says Durr, who co-directs the undergraduate Design Team program. “This team of undergraduates has been so successful because they created a practical solution for the problem after really understanding the constraints that needed to be met to be impactful.”
