All in the family — sodium and calcium ion channels share common roots
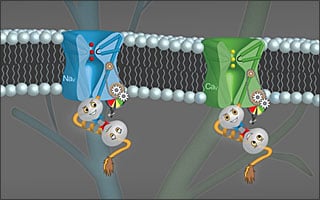
Sodium and calcium ion channels comprise two distinct types of molecules that are absolutely critical to our lives. Sodium channels conduct Na+ ions into cells, thereby initiating electrical signals that drive thoughts and heart beats. By contrast, calcium channels amplify the actions of these electrical signals by conducting Ca2+ ion flow into cells, which directly orchestrates brain communication and outright heart contraction.
Despite the distinctive designs of these molecules, a long-standing curiosity has been the eerie similarity of a portion of sodium and calcium channels. This similarity region perhaps hints at the presence of a common functional module in both sodium and calcium channels. For sodium channels, the purpose of this similarity region has long remained uncertain. By contrast, for calcium channels, the corresponding region supports an important form of biological feedback control, whereby rising Ca2+ levels in cells throttle the opening of calcium channels, thus maintaining an appropriate level of Ca2+. The similarity sequence binds to and works together with the Ca2+ sensing molecule calmodulin to accomplish this Ca2+ regulation, much as a temperature sensor and thermostat control household temperatures. When this Ca2+ feedback regulation goes awry, diseases like cardiac arrhythmias and Parkinson’s emerge, and there is an ongoing but thus-far-unrequited search for new drugs that could normalize this feedback control.
Out of this apparently diverse landscape comes a surprising yet fundamental unity in function amongst sodium and calcium channels, as described in a paper by the Calcium Signals Lab that appears in the 19 June 2014 issue of the journal Cell (paper entitled Conservation of Ca2+/calmodulin regulation across Na and Ca2+ channels). Lead author Manu Ben Johny, fellow Ph.D. graduate student Philemon Yang, Hopkins Biomedical Engineering Professor David T. Yue, and other members of the team ‘uncaged’ step-shaped elevations of Ca2+ near sodium channels. In so doing, they revealed that the similarity region of these channels also gives rise to a strong Ca2+-dependent regulation of sodium channel opening. Calmodulin also serves as the Ca2+-sensing element, and the molecular mechanism is uncannily similar to that in calcium channels.
The authors conclude that sodium and calcium channel families do harbor a common and vital Ca2+ feedback module, one that has persisted for more than a billion years of living history, when the designs of sodium and calcium channels first differentiated within the ancestors of the one-celled creature Paramecia. This likely lineage is artistically portrayed in the stylized phylogenetic branching of sodium and calcium channels.
The implications are vast. The newly revealed molecular mechanisms will critically aid the long search for pharmaceuticals that specifically target Ca2+ feedback regulation of these channels. The findings also comment on where ion channels came from, and highlight new approaches to disease that may emerge by viewing diverse sodium and calcium channels through the lens of a unified molecular perspective. In all, valuable treatment secrets to heart arrhythmias, skeletal muscle myotonias, and neurodegeneration may well reside within the common feedback module of sodium and calcium channel families.
